Area 1: Obesity and Diabetes
Gastric bypass surgery has emerged as the only potent and durable therapy for metabolic syndrome. Thus, we are interested in unravelling the mechanisms that mediate the therapeutic benefits of the surgery – with the long-term goal of developing novel potent and durable therapeutics. In this endeavor, we have demonstrated that Roux-en-Y gastric bypass (RYGB) leads to a profound metabolic and morphologic remodeling of the small intestine, which is manifested by significant increase in the intestinal glucose utilization and intestinal hyperplasia. It is our hypothesis that augmented intestinal glucose utilization plays a key role in improving type 2 diabetes following RYGB. Our current research endeavors aim to answer the following questions:
What is the overall contribution of the intestinal glucose utilization in improving type 2 diabetes?
While we, and others, have unanimously demonstrated that RYGB triggers a rapid and significant intestinal glucose utilization, it is still not clear whether and, to what extent, the intestinal glucose disposal contributes to the early glycemic control and long-term restoration of insulin sensitivity. We are using an array of molecular imaging, in vivo and ex vivo metabolic phenotyping, and transgenic animal models to mechanistically answer this question.
What are the underlying molecular and cellular mechanisms of the intestinal hyperplasia following RYGB?
The anatomically-reconfigured segments of the small intestine following RYGB (i.e., the Roux limb) undergo significant morphological remodeling. Those include increased villi density and height, and crypt depth, which are mirrored by the increased epithelial cell proliferation. More importantly, considering that proliferation is an energetically-demanding process, it is highly plausible that increased proliferation drives the augmented intestinal glucose need. However, still the cellular and molecular mechanisms that trigger the hyperplasia, or the relationship between the increased cellular proliferation and energy demand are not completely understood. Our approach to answer these questions includes application of in vitro model systems such as organoid, and novel transgenic and surgical animal models.
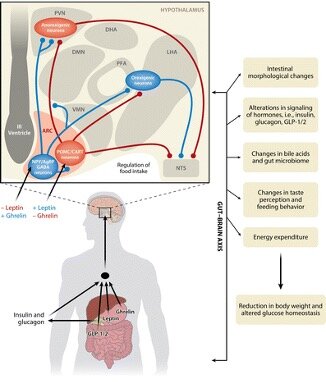
How does the multi-organ crosstalk drive the sustained effects of RYGB?
The metabolic impacts of RYGB spans multiple timescales and organs. For example, there is an immediate intestinal metabolic remodeling followed by a long-term restoration of peripheral insulin sensitivity. We are interested in understanding how the interactions among metabolically-active organs (e.g., gut, liver, adipose tissue, and muscle) drive the distinct physiological phases of the surgery. Our approach here heavily relies on multi-omics analysis of serum and tissues, and the development of sophisticated computational algorithms to integrate dynamic, multi-omics datasets. In this
endeavor, we are particularly interested in analyzing the portal vein, as it provides unique access to gut-bacterial derived metabolites and gut-liver-brain axis.
People:
AREA 2: MECHANO-METABOLISM
Mechanical stimuli play central role in a broad range of physiological processes including growth, differentiation and apoptosis. Indeed, impaired mechano-signaling is implicated in a number of pathophysiological conditions such as hypertrophic cardiomyopathy. We are interested in understanding how mechanical stimuli impact intestinal cell homeostasis. Toward this goal, we have developed a sophisticated cell culture platform that enables high fidelity interrogation of cellular responses to the substrate’s physicochemical composition. We are currently using this platform to answer the following questions:
How do the different intestinal cells perceive and respond to mechanical stimuli? And what are the molecular mechanisms underlying intestinal epithelial cell mechanosensing?
Using the aforementioned in vitro platform, we have demonstrated that substrate stiffness strongly regulates intestinal stem cells’ fate (i.e., their proliferation vs. differentiation propensity). We are currently determining the molecular cascades underlying the epithelial cell mechanosensing and signaling. In addition, we are interested in understanding how the peri-epithelial cells regulate fate and function of epithelial stem cells.
How does impaired mechanosensing contribute to certain intestinal diseases?
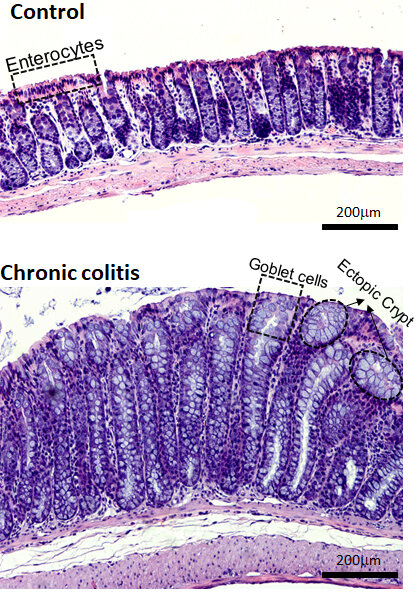
Several diseases of the small intestine, such as stenosis and Crohn’s disease are associated with excessive deposition of extracellular matrix (ECM) proteins. We are interested in investigating how the dysregulated mechanosignaling under those conditions contributes to the development or progression of the intestinal pathophysiological conditions. Our current focus is primarily on inflammatory bowel disease (IBD).
How could tissue biomechanics be used as a diagnostic tool?
A large number of diseases are associated with hyperactivation of mechanotransduction pathways and excessive accumulation of extracellular matrix proteins. Therefore, we are developing novel tools to non-invasively measure mechanical properties of hollow organs with the intent of utilizing them as diagnostic tools.
MechanoScopy™ elevator pitch video:
People:
Area 3: Microfabricated Systems
Intestinal epithelial cells are exposed to a broad range of chemical compounds (e.g., bacterial-derived metabolites, pathogens and their toxins, drugs, and food substances). We set out to investigate how these stimuli impact intestinal epithelial cells function and to understand their resilience and remodeling capacity. A high throughout microfluidic-based platform is the innate choice in this regard since it allows rapid and real-time interrogation of the cell response. We are currently focused on developing two technologies which offer two distinct capabilities:
Living Cell Array (LCA):
LCA enables simultaneous temporal expression profiling of multiple genes and their secrotome in a massively parallel, high throughput format. The overarching goal of this project is to develop a fully integrated microfabricated platform that would enable: a) long-term culture of intact primary intestinal epithelial cells in individual compartments, or cell chambers, of the microphysiological system; b) precise control of media and stimulants inflow with dynamic combinations into each cell chamber using integrated microvalve; c) quantitative live cell imaging of fluorescent protein transcriptional reporters; d) collection of outflow from each cell chamber for the downstream cell secretome analysis.
Gut-on-a-chip:
While, the LCA provides a powerful tool for real-time and high throughput interrogation of cell response to large number of chemical stimuli, in general, each experimental run can be performed using one type of cell. Therefore, LCA is not conducive to capturing interactions between multiple cell types – which is of significant biological importance. To address that limitation, we have developed a gut-on-a-chip model that is comprised of vertically stacked micro-fluidic channels that are separated with porous membranes. Using this design, we are able to co-culture cells in one chip (e.g., either a mixed population of cells on one membrane or different cell types on different membrane) and directly investigate their cross-interaction.